Inflammation and Helminths - Detail

Helminth modulation of the immune system
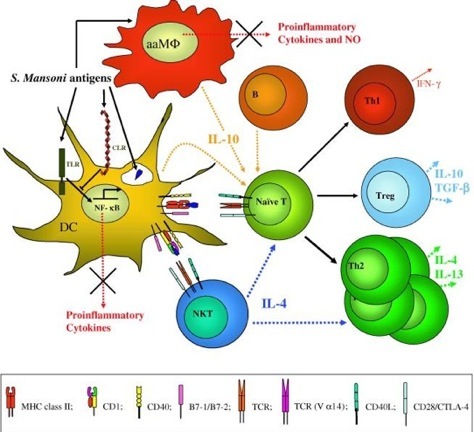
Amongst the various infectious agents, helminth parasites are regarded as master manipulators of the host immune system, often inducing a long-lasting asymptomatic form of infection (37,38). Parasitic worms can establish and reproduce in mammalian hosts, switching off the inflammatory immune response and inducing a tolerant response to parasite antigens. Following encounter with S. mansoni antigens, profound changes are observed in the innate immune system of the host, including modification of DC, MΦ, and NKT cells, phenotype and cytokine secretion (39,40). S. mansoni antigens can induce the secretion of regulatory cytokines from these cells as well as B1 B cells (41), resulting in the expansion of Th2 and Treg populations that might be responsible for maintaining self-tolerance (42–45) (see Figure 3).
Figure 3: S. mansoni modulation of the immune response. S. mansoni live helminth and antigens modify cells of the innate immune system through interaction with TLRs and CLRs arresting the production inflammatory mediators and eliciting instead, the release of immunoregulatory cytokines such as IL-10. This results in the generation of suppressive Treg and a bias towards a Th2 response. aaMΦ; alternately activated macrophage.
DC and MΦ are fundamental to directing immune responses along either a tolerating or activating pathway, therefore it is not surprising that helminths have evolved strategies targeting receptors on these cells. Toll like receptors (TLRs) and C-type lectin receptors (CLRs), broadly expressed on DCs and MΦs, are the main parasite targets for evading immuno-surveillance (46). More specifically, glycosylated molecules (expressed and secreted by S. mansoni) bind to the CLR and antagonize a TLR pro-inflammatory pathway (47). Numerous studies have shown that S. mansoni products induce IL-10 production by DCs and have a direct anti-inflammatory effect on DCs by controlling TLR ligand-induced DC maturation (48). S. mansoni has also been shown to induce alternatively activated MΦ, which secrete small amounts of inflammatory mediators and inhibit T cell proliferation (49).
The influence of helminth products on the innate immune system is not just restricted to DCs. Depending on the nature of the pathogen, NKT cells can direct the immune response in an appropriate direction by secreting a wide variety of pro- and anti-inflammatory cytokines (50). Schistosomes are rich in glycosylated molecules, which heavily decorate their integument or are actively secreted, and glycolipids presented by CD1d (a non-classical MHC molecule) on antigen presenting cells (APCs) may thus be able to activate regulatory NKT cells (51).
One of the most obvious and well-documented responses to S. mansoni is the Th2 dominance in the T cell population. Any initial Th1 response to the parasite is quickly redirected to a state of quiescence (52). The cytokine environment is fundamental for this purpose: large amounts of IL-4, IL-5 and IL-13 are secreted from the T cell pool, reinforcing not just T cell polarization, but also the anti-inflammatory loop on DC and MΦ (32). The parasite is also capable of containing the side-effects of such a strong Th2 response, inducing the secretion of IL-10 and TGF-β by other T cell subtypes (53,54). For example, animals and humans infected or exposed to S. mansoni antigens do not automatically develop allergies at a higher incidence (see Figure 3). The de novo induction and/or the expansion/recruitment of Treg almost certainly underlies the ability of many parasites to both evade a sterilizing immune response and also suppress both Th1 and Th2 arms of the adaptive immune system (55,56).
Parasitic worms and inflammatory diseases - continued
This paper was obtained from Medscape. Our understanding is that it because it was freely available that it is ok to publish it here with attribution. If this is not the case please let us know and we will remove it immediately.